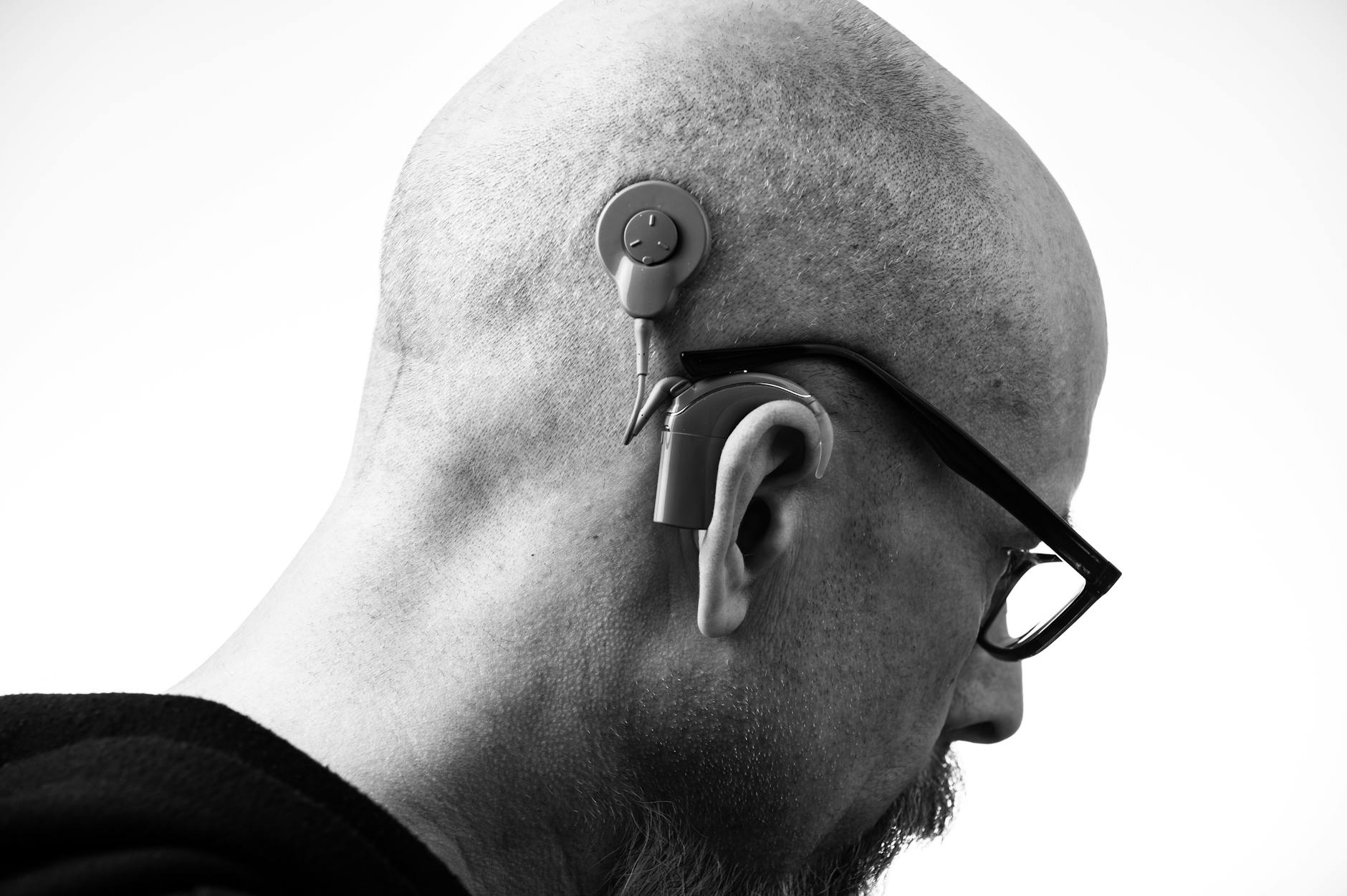
Lede: If you’ve ever read a headline promising “hearing restored in mice,” you’re not alone in wondering: when is it our turn? Here’s a clear-eyed tour of what scientists are actually doing to repair the inner ear, which breakthroughs are closest to helping humans, and what smart steps you can take now while the future unfolds.
First, why hair cells matter
Most permanent hearing loss in adults is sensorineural—the result of damage to tiny, sound-sensing cells in the cochlea (inner ear) and the synapses (connections) they make with the hearing nerve. Unlike skin or blood, the human inner ear doesn’t naturally regenerate these specialized structures once they’re lost. That’s why “regeneration” is such a big deal.
Meet the inner ear’s VIPs
- Outer hair cells (OHCs): Act like microscopic amplifiers, sharpening and boosting sound. They’re especially vulnerable to noise and certain medications.
- Inner hair cells (IHCs): Convert sound vibrations into electrical signals for the brain.
- Ribbon synapses: The tiny junctions where IHCs hand off signals to the auditory nerve. These can be damaged—even when the audiogram looks “normal.”
- Stria vascularis: A tissue that maintains the cochlea’s electrical “battery.” Age and vascular health can affect it.
To restore hearing meaningfully, researchers may need to replace or repair more than one of these parts. That’s the challenge—and the opportunity.
How do these cells get damaged?
- Loud sound exposure: Concerts, machinery, or even a single blast can injure hair cells and synapses.
- Age-related change (presbycusis): A mix of cumulative noise, metabolic stress, and genetics.
- Ototoxic medications: Some chemo drugs and antibiotics can harm hair cells (never stop a prescription without talking to your clinician).
- Inherited gene variants: Dozens of genes can influence inner-ear development or resilience.
Damage is often permanent today—but that’s exactly what regeneration research aims to change.
The main strategies scientists are testing
Think of inner-ear repair like fixing a delicate circuit board. Approaches range from swapping out broken components to rewiring connections.
1) Gene therapy: replacing or turning on the right instructions
Some types of hearing loss result from a missing or faulty gene. For those, delivering a healthy gene directly into the inner ear using a harmless viral carrier (often AAV) can, in theory, restore function.
- Single-gene deafness (near-term potential): Trials are underway in children with specific congenital forms (for example, variants that affect synaptic machinery). Early case reports in gene-specific conditions suggest partial restoration is possible for some individuals.
- Reprogramming support cells: Other trials aim to switch on developmental programs (like the transcription factor ATOH1) so supporting cells could convert into hair cells. So far, translating success from animals to humans has been difficult—dosage, targeting, and timing are complex.
What to watch for: gene therapy currently makes the most sense for well-defined, single-gene causes—especially identified early in life. For common age- or noise-related loss, multiple pathways are involved, so a simple “gene swap” is less likely to be the answer.
2) Small molecules: coaching the ear to self-repair
Another path uses drugs that nudge inner-ear cells back into a growth-and-differentiation mode, often by modulating the Notch and Wnt pathways—two molecular switches critical in hair cell development.
- Hair cell regeneration in adults: Several companies have tried combinations that showed promising animal data. High-quality human trials so far have not delivered the consistent, meaningful hearing improvements we hoped for, especially on speech-in-noise measures. Many programs have been paused or redirected after negative results.
- Synapse repair (for “hidden hearing loss”): A line of research focuses on regrowing or protecting the ribbon synapses between inner hair cells and the auditory nerve. Early-stage human studies are mixed; some show hints of benefit, others do not. Dosing, delivery (often via injection through the eardrum), and choosing the right patients remain hurdles.
Bottom line: small molecules remain attractive because they’re potentially repeatable and adjustable. But we need better targeting, biomarkers, and trial designs.
3) Stem cells and cell replacement: building new parts
In the lab, scientists can now coax stem cells into hair cell–like cells that respond to sound. The dream is to transplant these into a damaged cochlea and have them wire up correctly.
- Big wins in dishes and animals: We can grow hair cell–like cells and even organoids (mini inner ears) for testing.
- The catch in humans: Getting cells into the tightly coiled cochlea, ensuring they survive, align, and integrate into the existing circuitry safely—and do so in a way that actually improves how you hear conversations—remains a steep climb.
So, exciting science? Absolutely. Ready for clinic? Not yet.
4) Neuroprotection and anti-inflammatory approaches
Because not all damage is a clean “on/off” event, protecting vulnerable cells during stress (noise, illness, ototoxic exposure) is a hot area. Antioxidants, ion channel modulators, and anti-inflammatory agents are under study.
Practical point: these aren’t “regeneration,” but if they reduce injury or stabilize function during an acute risk window, they can preserve more of what you have—an underappreciated win.
5) Beyond biology: smarter devices and hybrid futures
While biology advances, cochlear implants and hearing aids keep getting better at delivering speech in complex environments. Research is exploring:
- Optical/electrical stimulation: Using light to stimulate the auditory nerve for finer frequency resolution (very early-stage).
- Closed-loop devices: Systems that sense your listening environment or brain responses and adapt in real-time.
Devices won’t regenerate cells, but they may bridge the gap—and even stay part of care if regenerative therapies arrive.
How close are we, really?
Here’s the responsible, not-depressing truth: progress is real, but uneven.
- Near-term (0–5 years): The most realistic wins are in single-gene early-onset hearing losses via gene therapy, and potentially targeted synapse-protection or -repair therapies for specific groups.
- Medium-term (5–10 years): Better biomarkers (like objective synapse measures), refined drug delivery to the cochlea, and combination strategies may finally show consistent speech-in-noise gains in trials.
- Long-term (10+ years): True hair cell replacement that integrates and improves natural hearing across pitches in adults remains a moonshot—but not science fiction.
Timelines in medicine can shift in both directions. That’s why understanding the evidence—and taking care of the hearing you have now—matters.
How to read the headlines without whiplash
- Check the trial phase: Phase 1 tests safety; Phase 2 looks for signs of benefit; Phase 3 asks “does this work reliably in many people?” Early “breakthroughs” often fade under larger, blinded testing.
- Look at the endpoints: Did hearing improve on speech-in-noise or just on a tonal audiogram? Did tinnitus or listening effort change? Functional outcomes matter most.
- Size and controls: Was there a placebo/sham group? How many people were studied? Small, open-label trials are useful for signals but not for proof.
- Population fit: Was the study in congenital, noise-induced, or age-related loss? Results rarely generalize across causes.
- Peer review and registration: Is the trial listed on a registry (like ClinicalTrials.gov)? Has data been published in a peer-reviewed journal?
What you can do now (while the labs keep cooking)
1) Guard the hearing you have
- Turn it down, walk away, plug up: Keep volume under 60% for personal audio, step back from speakers, and use high-fidelity earplugs at concerts or loud work sites.
- Mind medications: If you’re prescribed a potentially ototoxic drug, ask your care team about monitoring. Don’t stop medications on your own.
- Heart and head health: Manage blood pressure, diabetes, and sleep—your ears are vascular and neural organs too.
2) Treat what’s treatable
- Don’t wait on hearing support: Well-fit hearing aids or cochlear implants can drastically improve speech understanding, reduce listening effort, and keep you engaged. Early adoption is linked to better outcomes.
- Verify the fit: Ask for real-ear measurements with hearing aids, and comprehensive counseling for features that help you in noise.
- Rehabilitation helps: Auditory training and communication strategies can boost real-world outcomes beyond devices alone.
If you’re noticing difficulty, a licensed audiologist can map your hearing, discuss options, and help monitor changes over time. Consider it baseline data for your future self.
3) Stay informed—without chasing miracles
- Follow reputable sources: University trials, national institutes, and peer-reviewed journals beat social media hype.
- Ask about eligibility: Some clinical trials need volunteers; your audiologist or ENT can help you understand if a study matches your profile.
- Keep receipts (of your data): Audiograms, speech-in-noise scores, and tinnitus questionnaires help you track meaningful changes.
Common myths, kindly debunked
- “A supplement can regrow hair cells.” There’s no high-quality human evidence that over-the-counter supplements can regenerate cochlear hair cells. Some nutrients support general health; none replace lost sensory cells.
- “If regeneration is coming, I’ll wait to treat my hearing.” Untreated hearing loss can strain communication, increase listening effort, and limit participation now. Using appropriate devices and strategies today doesn’t disqualify you from tomorrow’s options.
- “One cure will work for everyone.” Hearing loss is many conditions. Regeneration may arrive first for specific genetic or injury patterns—not as a one-size-fits-all fix.
Bring this to your next appointment
- “Based on my audiogram and history, what’s the likely cause of my hearing difficulties?”
- “Would speech-in-noise testing or tinnitus assessment give us a fuller picture?”
- “Am I a candidate for any current treatments or clinical trials?”
- “What can we do now to protect and maximize the hearing I have?”
Curious about where you stand? A gentle nudge: schedule a hearing evaluation with a licensed audiologist. It’s empowering to know your baseline—and it puts you in the driver’s seat as new therapies emerge.
Further Reading
- Sudden Hearing Loss? Don’t Wait—The 72‑Hour Window That Can Save Your Hearing (Hearing Loss) - Sudden Hearing Loss: The 72-Hour Treatment Playbook (What to Do Now) (Treatment) - Sudden Hearing Loss Needs Speed: Treatments and the Critical Window (Treatment) - Your Heart, Your Hearing: The Cardiometabolic Link You Can’t Afford to Ignore (Research)Frequently Asked Questions
Will hair cell regeneration also fix tinnitus?
It depends on the cause. Tinnitus can stem from multiple sites along the auditory system, and sometimes from the brain’s response to reduced input. If a future therapy restores useful signal where damage occurred, some people may experience tinnitus relief—but others may not. Today, evidence-based tinnitus management focuses on sound therapy, counseling, and addressing co-factors like sleep and stress. An audiologist can tailor options to you.
Are any regeneration treatments available to the public right now?
No widely available, proven hair cell regeneration treatments exist yet for common adult hearing loss. A few gene therapies for specific, single-gene childhood conditions are in early clinical trials, and research into synapse repair and protective drugs continues. For most adults, the best-supported options today are well-fit hearing aids, cochlear implants when appropriate, and rehabilitative strategies.
If I’m older, could I still be eligible for future therapies?
Possibly. Eligibility depends less on age and more on the type and extent of your inner-ear damage, your general health, and the specific therapy’s design. Some approaches may target synapses or inflammation rather than creating new hair cells, which could be relevant at many ages. Staying engaged in routine hearing care keeps your records current for any future opportunities.
How can I tell if a headline about hearing restoration is credible?
Look for studies registered on ClinicalTrials.gov, published in peer-reviewed journals, with clear endpoints (especially speech understanding), control groups, and adequate sample sizes. Beware of testimonials, non-specific claims, and products sold directly to consumers promising “regrowth.” When in doubt, bring the article to your audiologist or ENT and ask for their take.