Lede: Imagine fixing hearing not just with microphones and microchips, but by repairing the ear itself—regrowing the tiny sensory cells and reconnecting broken synapses. That future is inching from sci‑fi toward real clinical trials. Here’s a friendly, hype-free tour of what’s happening, who might benefit first, and how to stay ready without putting your life on hold.
The bottleneck: When the cochlea’s tiny parts wear out
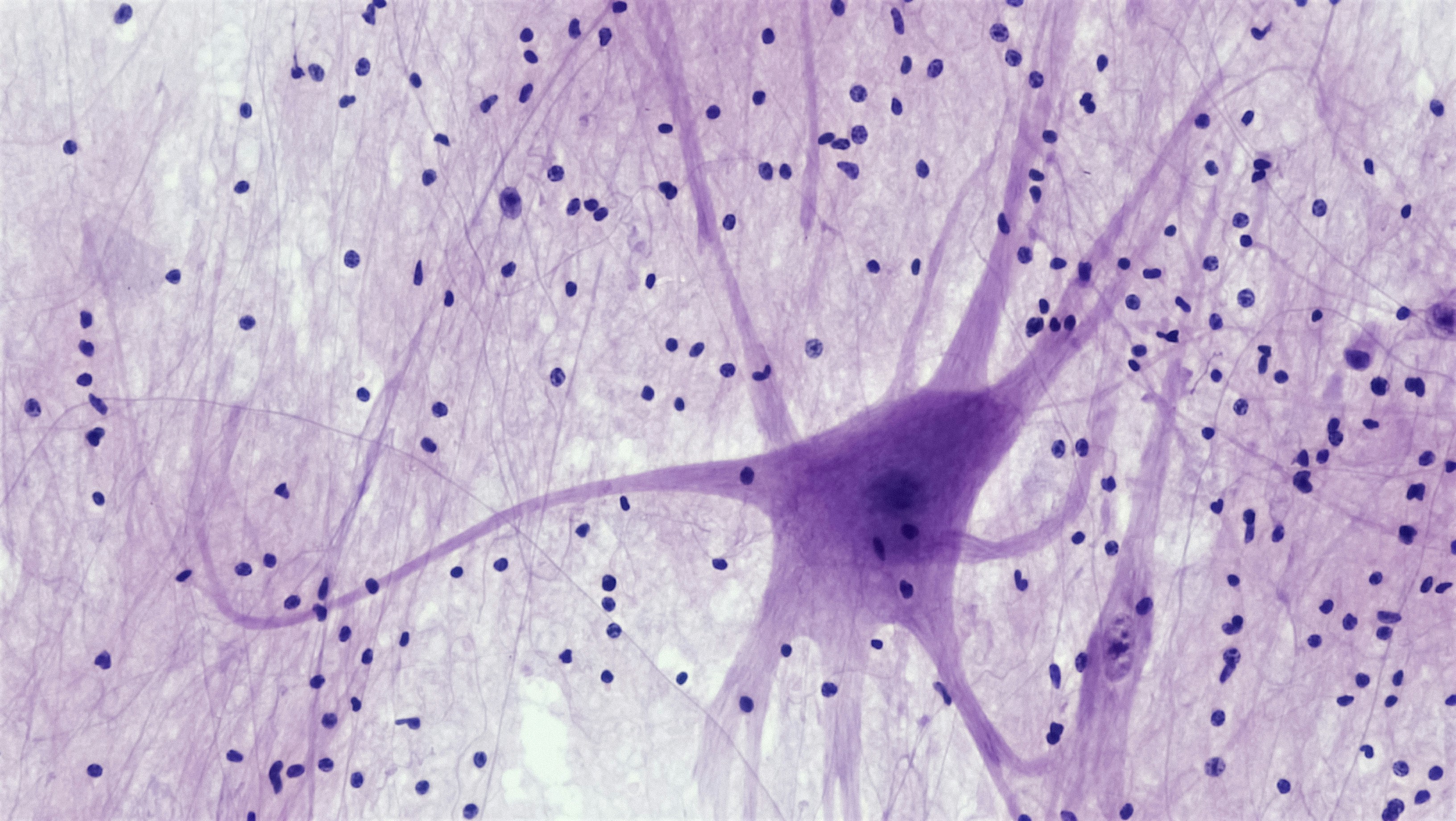
Most permanent (sensorineural) hearing loss starts in the cochlea, a spiral structure in the inner ear. Two key players there matter for sound clarity:
- Hair cells — microscopic “microphone” cells that convert sound vibrations into electrical signals.
- Synapses and auditory nerve fibers — the wiring that carries those signals to the brain.
Noise, aging, some medicines, and certain genes can damage hair cells and the synapses that connect them to the auditory nerve. If you’ve ever felt that voices are loud but unclear—especially in noise—that’s often a synapse story as much as a hair cell story.
Hair cells vs. synapses vs. nerve: A 20‑second primer
- Lose outer hair cells, and sounds lose crispness; you need more volume and better fine-tuning.
- Lose inner hair cell synapses, and speech-in-noise gets hard, even if a basic tone test looks okay.
- Lose nerve fibers, and the brain gets a weaker, fuzzier signal overall.
Why fish can regrow hearing… and we (mostly) can’t—yet
Birds and fish can regenerate hair cells after damage. Mammals, including humans, generally can’t. Our cochlear “supporting cells” lose their regenerative mojo after development. The big scientific puzzle has been how to coax those supporting cells to become new, working hair cells—and then reconnect them to the nerve in the right pattern.
Over the last decade, researchers have uncovered the genetic switches that control this process (think ATOH1 and Notch/Wnt signaling), and they’re learning how to rekindle them in adult inner ears. In animals, that’s produced stunning lab results. The challenge is translating those wins to the much more delicate, tiny human cochlea, safely and consistently.
The main approaches scientists are testing
1) Pharmacologic hair cell regeneration (small molecules)
Goal: Turn on pathways that nudge supporting cells to divide and transform into hair cells.
- What’s been tried: Small‑molecule drug combinations targeting Notch/Wnt pathways. Some early clinical programs showed promise in animals but mixed or negative results in human trials so far.
- Where it stands: Research continues, but no approved medicine yet. Lessons learned are guiding better drug delivery (getting the right dose across the round window membrane) and smarter patient selection (matching the drug to the kind of damage a person has).
Takeaway: This path isn’t dead; it’s maturing. Expect more precise targets, better delivery gels, and trials that enroll people whose inner ears are most likely to respond.
2) Gene therapy for specific, single‑gene hearing loss
Goal: Replace or repair a faulty gene in the inner ear so hair cells—or their synapses—work again.
- Who it’s for first: Children with monogenic hearing loss (caused by a mutation in a single gene), such as OTOF (otoferlin) deficiency. In OTOF loss, hair cells are present but can’t release neurotransmitter to the nerve. Fix the gene, and the connection may be restored.
- What’s happening: Early human trials using adeno‑associated virus (AAV) to deliver a working gene into the cochlea are underway for OTOF and other targets. Initial reports from some centers suggest measurable improvements in hearing in select children, but data are still limited and follow‑up is short.
- How it’s done: A one‑time inner‑ear injection under the eardrum or via a tiny surgical opening. It’s delicate, performed by otologic surgeons.
Takeaway: This is the most advanced hearing-restoration strategy right now, but it’s not a one‑size‑fits‑all fix. It’s tailored to specific genes, mostly in pediatric patients.
3) Synapse repair (cochlear “rewiring”)
Goal: Rebuild the connections between inner hair cells and the auditory nerve—especially relevant for noise‑exposed ears with speech‑in‑noise struggles.
- The tools: Neurotrophins (like NT‑3 and BDNF) and other molecules that invite nerve fibers back to hair cells and stabilize synapses.
- Progress: Strong animal data show repaired synapses and improved neural signaling. Human work is in early stages, with efforts focusing on safe delivery and dosing.
Takeaway: If your audiogram looks mild but conversations are exhausting, this research line is one to watch.
4) Cell replacement and stem cell approaches
Goal: Grow brand‑new hair cells or even auditory neurons from stem cells or by reprogramming supporting cells.
- What we know: Scientists can now grow hair‑cell‑like cells in the lab and even organoid “mini‑cochleas.” Translating that into precise, safe, in‑ear replacement is the next hurdle.
- Risks to manage: Misplacement, overgrowth, or cells that don’t wire up correctly. Safety and control are paramount.
Takeaway: Powerful in concept, early in humans. Likely to pair with synapse repair to make new cells actually talk to the brain.
5) Next‑gen devices: Optogenetic cochlear implants
Goal: Combine biology with engineering—make auditory neurons respond to light and drive them with tiny LEDs instead of electrical current. Light can be steered more precisely than electricity, potentially improving pitch resolution.
- Status: Animal studies are promising; human feasibility work is beginning. This would still involve surgery and a device, but could offer more natural sound in the long run.
Takeaway: Think of this as a “biotech‑boosted” cochlear implant, not a cure. It’s exciting but still experimental.
What this could mean for you (and when)
Who may benefit first
- Children with a known single‑gene cause of severe to profound hearing loss, particularly where hair cells are present but can’t signal correctly (e.g., OTOF).
- Adults with specific genetic variants could be next as programs expand beyond pediatric populations and targets broaden.
- Noise/age‑related loss may eventually benefit from synapse‑repair drugs or combination approaches, but timelines are less certain.
Wherever you are on the journey, staying connected to an audiologist or ENT can help you track trials, eligibility, and smart next steps. They can coordinate genetic testing when appropriate and ensure your current hearing tech is optimized so you’re not waiting in silence for tomorrow’s science.
Safety, durability, and unknowns
- Durability: We don’t yet know how long gene therapies last in the inner ear. Years? Decades? Redosing might be limited by immune responses.
- Precision: Too much cell growth or growth in the wrong place would be harmful; delivery methods are designed to minimize that risk.
- Eligibility: Many trials have strict age, hearing, and genetic criteria. Early studies often prioritize children to rescue hearing during critical language windows.
Practical expectations: A realistic timeline
- Near term (0–3 years): More data from pediatric gene therapy for select single‑gene causes; refined surgical delivery; small, carefully controlled expansions.
- Medium term (3–7 years): Additional monogenic targets; first human studies of synapse‑repair agents in broader adult populations; better drug delivery systems.
- Longer term (7–15 years): Combination strategies (regrow cells + reconnect synapses); earlier optogenetic implant studies; progress in cell replacement with tighter safety controls.
Important: Timelines can speed up—or slow down—based on trial outcomes. Science is iterative. Negative trials teach us where to aim next.
How to stay ready without waiting on the sidelines
- Protect what you have: Use hearing protection in loud environments and mind your daily noise dose. Prevention still beats repair.
- Optimize today’s tech: Well‑fit hearing aids or cochlear implants keep your brain’s hearing pathways active, which may make future therapies more useful. Ask your audiologist about features that reduce listening effort.
- Consider genetic testing if hearing loss started early in life or runs in the family. Your clinician can guide you to appropriate, ethical testing—and help you understand results.
- Follow legitimate trials: ClinicalTrials.gov lists registered studies. Discuss any trial you’re considering with your audiologist or ENT to weigh risks and benefits.
- Take care of whole‑body health: Heart health, diabetes control, sleep, and stress management all influence hearing resilience and how you’ll do with any treatment.
If you already use hearing aids or a cochlear implant
Future biological treatments aren’t an “either/or” with devices. They could complement each other:
- Hearing aids: If synapse repair improves clarity, you may benefit from new programming with less gain but better speech‑in‑noise performance.
- Cochlear implants: Gene or cell therapies might preserve or even enhance residual hearing, enabling hybrid acoustic + electric strategies. Optogenetic implants would still be implants—just with potentially finer pitch detail.
Practical tip: Keep your routine follow‑ups. Ask your audiologist to document stable baselines (audiogram, speech scores). Good records make it easier to spot meaningful changes and evaluate eligibility if a trial opens.
Ethics and participation: Questions to ask before joining a trial
- What is the main goal—safety, dose‑finding, or efficacy?
- What are the known risks and unknowns? How will complications be handled?
- What happens if I need future treatments—will this limit options?
- How will success be measured (audiograms, speech tests, real‑world outcomes)?
- Is there support (e.g., travel, follow‑up care) for families?
If you’re a parent considering a pediatric trial, it’s okay to take your time, bring questions, and seek a second opinion. Your audiologist and ENT specialist can be your coaching staff.
Bottom line
Regenerating hearing is no longer a fantasy—it’s a work‑in‑progress with early wins in select genetic conditions and promising paths for synapse repair. For most adults with age‑ or noise‑related loss, today’s best bet is still excellent fitting, smart hearing protection, and staying plugged into the research. Keep your expectations realistic and your options open. And keep your audiologist on speed dial—they’re your guide for what’s real, what’s next, and what’s right for you.
Gentle next steps
- Haven’t had a hearing test in a while? Book an audiology visit to establish a clean baseline.
- Curious about your candidacy for future therapies? Ask your clinician whether genetic testing makes sense in your situation.
- Feeling overwhelmed by the science? Bring this article to your appointment and talk it through together.
Further Reading
- Can We Regrow Hearing? Inside Hair-Cell, Synapse, and Gene Therapies Now (Research) - Can We Regrow Hearing? The Real State of Hair Cell Regeneration (Research) - Sudden Hearing Loss? Don’t Wait—The 72‑Hour Window That Can Save Your Hearing (Hearing Loss) - When Sound Fades Overnight: The Hearing Emergency You Should Never Ignore (Hearing Loss)Frequently Asked Questions
Will gene therapy make hearing aids obsolete?
Not in the near term. Early gene therapies target specific single-gene causes, mostly in children. Most adults with age- or noise-related loss will still rely on hearing aids or cochlear implants for the foreseeable future. Biological treatments may eventually complement devices rather than replace them outright.
Can adults with age-related hearing loss get hair cell regrowth soon?
There’s no approved hair cell–regrowth treatment for age-related hearing loss yet. Research is active on synapse repair and pharmacologic regeneration, but timelines are uncertain. Keep your hearing protected and your current technology optimized while the science advances.
Is CRISPR being used to fix hearing?
CRISPR is being explored in the lab and early research settings for some genetic hearing conditions, but most current human inner-ear trials use AAV gene delivery to add a working gene rather than directly editing DNA. It’s a rapidly evolving field, and safety is the top priority.
How safe are inner-ear gene or cell therapies?
So far, safety data in small early trials look cautiously encouraging, but questions remain about durability, immune responses, and rare complications. Procedures are done by specialized surgeons. If you’re considering a trial, review risks, benefits, and follow-up requirements with your ENT and audiologist.