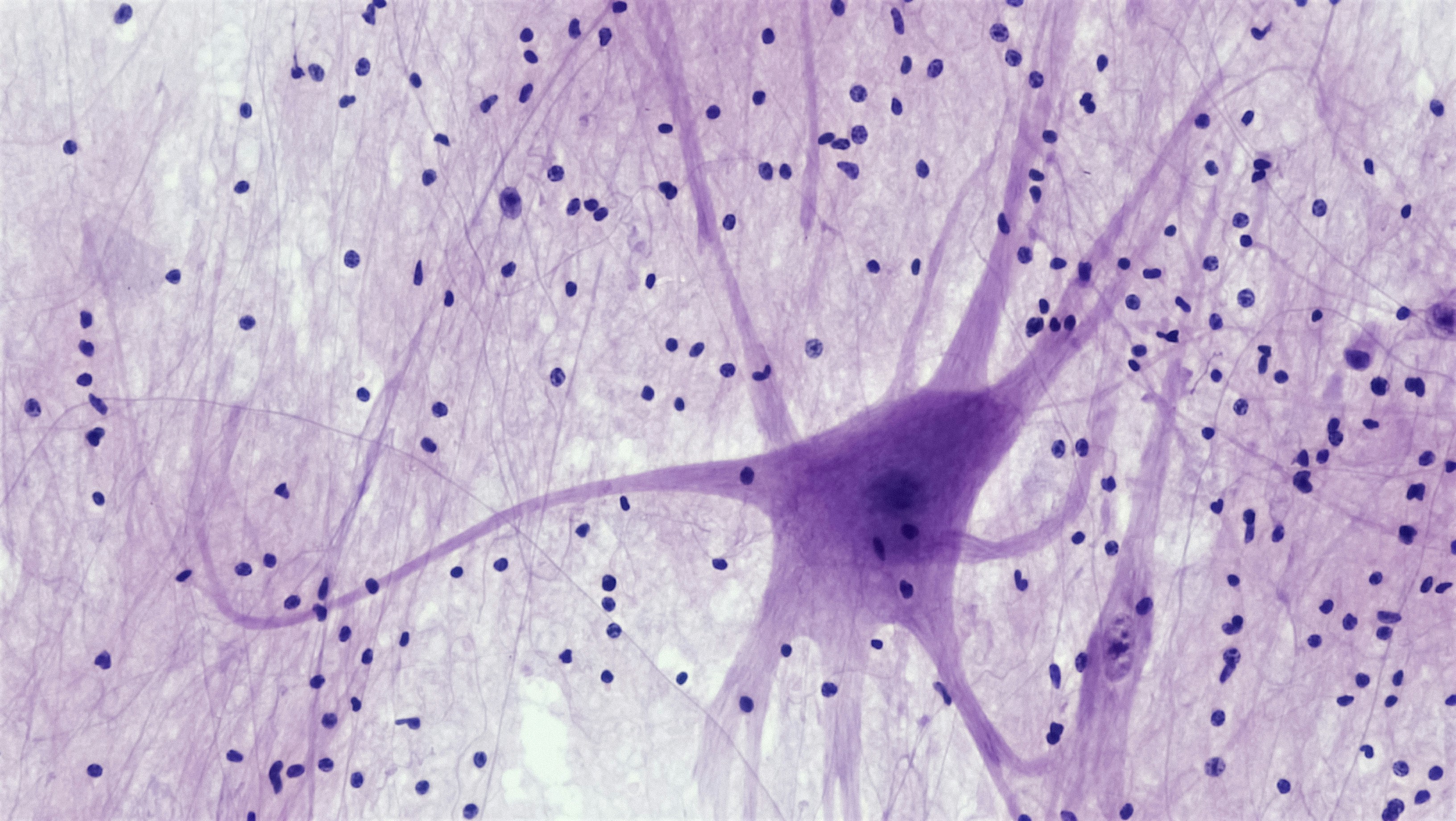
If you could zoom into your inner ear, you’d see a delicate forest of hair cells and nerve endings converting tiny vibrations into electricity your brain understands as sound. When those cells and connections are lost, hearing fades. The big research question: can we bring them back?
Short answer: progress is real, but uneven. We have early wins in very specific genetic forms of deafness, better protection for some patients at high risk, and clearer roadmaps for repairing the ear’s wiring. The blockbuster cure for age‑related or noise‑related hearing loss isn’t here yet. But the science is moving, and there’s a lot you can do right now to protect and optimize the hearing you have.
A 60‑second tour: what actually breaks in sensorineural hearing loss
“Sensorineural” usually means damage inside the cochlea or the auditory nerve. Several parts can fail:
- Outer hair cells (OHCs): tiny amplifiers that make soft sounds audible and sharpen tuning.
- Inner hair cells (IHCs): the main sensors that send sound info to the brain.
- Synapses (the IHC–auditory nerve connections): the plugs where signals jump to the nerve. These can be lost even when the audiogram looks “normal,” a pattern often called hidden hearing loss.
- Auditory nerve fibers: the cables carrying the message to the brain.
- Stria vascularis/metabolic support: the cochlea’s “battery” that keeps the whole system powered.
Different causes—loud sound, aging, certain drugs, infections, genetics—can damage one or more of these targets. That’s why one person struggles in noise while another loses high pitches first, and another hears fine but has constant ringing.
Two main routes to restoration
1) Regrow or replace lost hair cells
Mammals don’t spontaneously regrow cochlear hair cells. Researchers try to “flip the switch” by pushing supporting cells to become hair cells using developmental genes and pathways (like ATOH1, Notch, and Wnt). In animals, this can create hair‑cell‑like cells and partial recovery of function. In humans, first‑generation small‑molecule approaches have not yet produced reliable, meaningful hearing improvement in controlled trials.
2) Repair the wiring (synapse and neuron rescue)
Even with hair cells present, the synapses that connect them to nerve fibers can be wiped out by noise or aging. Lab studies show that growth factors (e.g., neurotrophins) can coax new synapses to form in animals and restore some neural responses. Translating that to humans has been challenging: finding the right drug, dose, timing, and delivery to the right part of the cochlea.
What’s working in people (so far)
- Gene therapy for single‑gene deafness: For children born without a key synaptic protein (for example, mutations in OTOF that block synaptic release at inner hair cells), early trials using AAV (adeno‑associated virus) to deliver a working gene have reported meaningful restoration of sound detection and speech access. These are small, early studies, but it’s a milestone: repairing the “wiring” at the synapse so signals can flow.
- Protection in high‑risk settings: Sodium thiosulfate, when used in carefully timed protocols for children receiving cisplatin chemotherapy, can reduce the risk of hearing loss without undermining cancer treatment. Prevention isn’t sexy, but it’s powerful.
- Better delivery tools: Ear surgeons and researchers have refined microsurgical and intratympanic delivery to reach the cochlea more evenly and safely, crucial for any future regeneration or repair drug.
On the flip side, several high‑profile programs aiming to regrow hair cells or repair synapses in acquired hearing loss have not met primary endpoints in controlled trials. That’s not failure—it’s feedback. The field is recalibrating doses, timing, and targets, and learning that human ears are a tougher puzzle than mouse ears.
Why it’s hard (and why that’s okay)
- Timing matters: Fresh damage can be rescued more readily than long‑healed scars. Acute noise or drug injury presents a small therapeutic window; decades‑old hearing loss is harder.
- Delivery is surgical: The cochlea is tiny, spiral, and fluid‑filled. Getting the right concentration of medicine to the right turn without harming tissue is an art and a science.
- Multiple failure points: A person with age‑related loss might have hair‑cell damage, synapse loss, metabolic decline, and neural changes—all at once. One drug won’t fix everything.
- Measuring success is tricky: Standard audiograms miss synapse damage and don’t capture speech‑in‑noise very well. Trials increasingly include speech‑in‑noise and objective electrophysiology, but agreeing on clinically meaningful change is complex.
- Safety and durability: We need improvements that last without triggering inflammation or off‑target effects, especially for gene therapies.
Who might benefit first
- Children with single‑gene causes of auditory synapse dysfunction (like OTOF): Early wins are already emerging here because the target is clear and the ear hasn’t undergone decades of degenerative change.
- Acute injuries (certain ototoxic exposures or sudden synaptic insults): If we can deliver protective or repair agents quickly, there’s a logic for better outcomes.
- Broader adult hearing loss (noise, aging): This group stands to gain enormously, but solutions likely require combination strategies—protect the remaining cells, repair synapses, and possibly stimulate regeneration—plus rehabilitation via hearing devices and training.
What you can do now while science advances
You don’t have to wait for a lab breakthrough to improve how you hear and live today.
- Get a baseline hearing test: Knowing your audiogram, middle‑ear status, and speech‑in‑noise performance helps you and your clinician track change and catch treatable issues early. If you’ve never had a comprehensive hearing evaluation, consider booking one with an audiologist.
- Turn down risk: Noise is still the biggest modifiable threat. Use well‑fitting earplugs or earmuffs at concerts, in loud workplaces, and during power‑tool use. Keep personal listening devices at safe volumes and limit exposure time.
- Guard your “cochlear battery”: Cardiovascular health supports the stria vascularis and blood supply to the ear. Managing blood pressure, diabetes, and smoking status benefits your hearing as well as your heart.
- Act on hearing difficulty sooner, not later: Hearing aids and assistive tech don’t just make things louder—they improve clarity, reduce listening effort, and help keep the brain’s speech networks engaged. Evidence suggests that treating hearing loss may help reduce cognitive decline risk in older adults.
- Ask about candidacy for implants or hybrid devices: For severe loss, cochlear implants can restore access to speech when hearing aids can’t. Hybrids combine acoustic amplification for lows with electrical stimulation for highs.
- Consider research participation: If you’re curious and eligible, clinical trials advance the field. Talk with your audiologist or ENT about reputable registries.
How to read a “hearing breakthrough” headline
Here’s a quick filter to separate hype from hope:
- What phase is the study? Animal, early human safety (Phase 1), or controlled efficacy (Phase 2/3)?
- How many people and how long? Single‑patient case report or randomized, blinded trial with months of follow‑up?
- What changed? Just a pure‑tone threshold, or also speech‑in‑noise, objective neural measures, and patient‑reported outcomes?
- Durability and safety? Are gains stable? Any inner‑ear inflammation or balance issues reported?
- Is it peer‑reviewed and replicated? Preprints are useful, but independent replication builds trust.
Peeking over the horizon
The most exciting ideas being stress‑tested now include:
- Combination therapies: Pairing synapse repair with hair‑cell support, or adding anti‑inflammatory agents to create a healing environment.
- Gene editing and RNA approaches: CRISPR‑based and antisense strategies for specific mutations (TMC1, GJB2, and others) are advancing in animal models and early clinical explorations.
- Next‑gen delivery: More targeted AAV capsids, round‑window smart gels, and micro‑pumps to achieve even distribution without surgery.
- Better diagnostics: Objective measures of synaptopathy and auditory nerve health could match patients to the right treatments and detect change earlier than audiograms.
The bottom line: for broad, acquired hearing loss, restoration will likely arrive stepwise, starting with specific subgroups, then expanding as we learn to combine repair strategies. In the meantime, prevention and rehabilitation remain incredibly effective.
If you’re noticing changes in your hearing, tinnitus, or sound tolerance, a licensed audiologist can help you map out a personalized plan—protection, testing, devices, and (if appropriate) referrals for medical care or research opportunities.
Further Reading
- Can We Regrow Hearing? Inside Hair-Cell, Synapse, and Gene Therapies Now (Research) - Can We Rebuild Hearing? The Real State of Hair Cell Regrowth and Gene Therapy (Research) - Can We Regrow Hearing? The Real State of Hair Cell Regeneration (Research) - Hearing Damage, Measured in a Drop: The Emerging Science of Ear Biomarkers (Research)Frequently Asked Questions
When might adults with age‑related or noise‑related hearing loss see regenerative treatments?
It’s hard to set a date. Early human successes have come in narrowly defined genetic conditions. For common adult hearing loss, researchers still need to solve delivery, target selection, and measuring meaningful benefit. Many experts expect progress to roll out in stages over years rather than a single cure arriving all at once. While we wait, hearing conservation and timely rehabilitation with hearing tech can dramatically improve day‑to‑day communication.
If regeneration arrives, will hearing aids become obsolete?
Unlikely any time soon. Hearing aids, cochlear implants, and assistive listening systems are proven, adjustable, and available now. Even as biological therapies emerge, many people will still benefit from devices—alone or in combination—to optimize clarity in real‑world settings. Think of future care as a toolkit, not a single tool.
Can supplements regrow hair cells or fix synapses?
There is no high‑quality evidence that over‑the‑counter supplements can regenerate hair cells or repair auditory synapses in humans. Some nutrients support overall health, but claims of hearing restoration warrant healthy skepticism. Before starting any supplement, discuss it with a healthcare professional, especially if you take other medications.
How do I find trustworthy clinical trials for hearing restoration?
Start with major registries like ClinicalTrials.gov and academic medical centers with otology programs. Look for trials with clear inclusion criteria, defined endpoints (not just testimonials), and safety monitoring. An audiologist or ENT can help you understand eligibility and potential risks and benefits.