Lede: The most common question in hearing clinics—after "Do I really need hearing aids?"—is "Are scientists close to restoring hearing?" Short answer: for some specific genetic conditions, early gene therapy results are genuinely exciting. For age-related or noise-induced hearing loss, we’re not there yet. But research is moving fast, and knowing what’s real (and what’s hype) can help you plan your hearing health with confidence.
The big idea: What actually breaks in sensorineural hearing loss
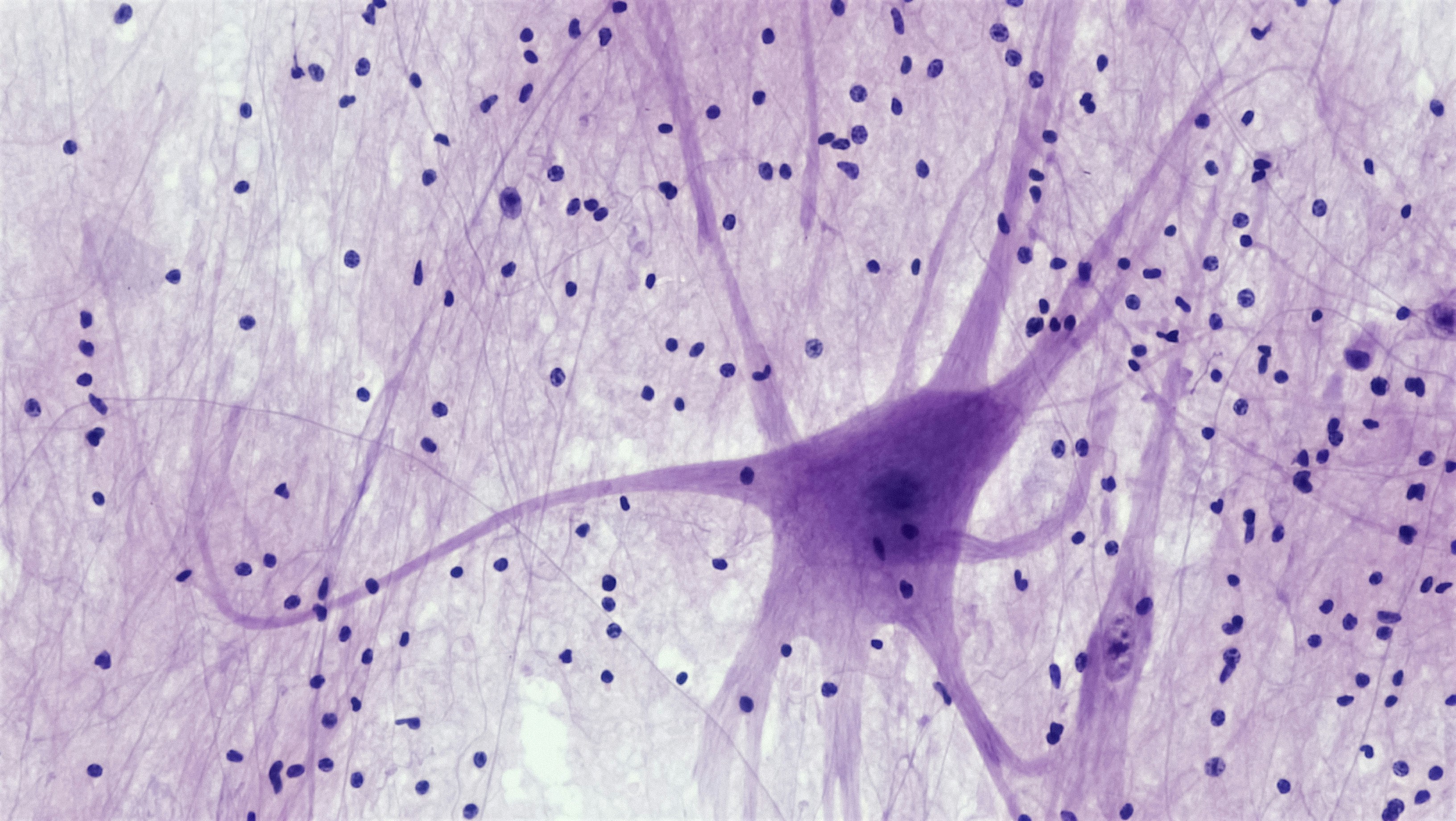
Most permanent hearing loss happens in the inner ear—the cochlea. Two tiny players do the heavy lifting:
- Hair cells: The cochlea’s sound sensors. Outer hair cells amplify and fine-tune, inner hair cells send the signal to the brain.
- Synapses (ribbon synapses): The microscopic connections between inner hair cells and the hearing nerve (auditory nerve) fibers.
With age, loud noise, some medications, and certain infections or genes, these structures can be damaged:
- Hair cells die and don’t naturally regrow in humans.
- Synapses break—you may still hear tones on an audiogram but struggle with speech in noise (often called “hidden hearing loss” in research).
So, research is aiming at three frontiers: protect/repair synapses, regrow hair cells, and replace missing genes in certain inherited forms.
Frontier #1: Repairing the “hidden” damage—synapses
When people say “I hear, but I can’t understand,” synapse loss is a prime suspect. Animal studies show noise can strip synapses without wiping out hair cells. Could we reconnect those lines?
What scientists are trying
- Neurotrophins (like BDNF or NT-3) and related molecules: These growth factors help nerves and synapses survive and reconnect in lab and animal models.
- Small molecules: Designed to protect or regrow the hair cell–nerve connection.
Where the evidence stands
- Several clinical programs tested inner-ear injections aiming to improve speech-in-noise by repairing synapses. A few advanced trials have not shown the consistent benefit researchers hoped for to date, and some programs were discontinued. That’s disappointing—but also informative, refining doses, delivery, and outcome measures.
- Key takeaways: Synaptopathy is real in animals; measuring it and reversing it in humans is harder than it sounds. Expect more targeted trials using better biomarkers and more sensitive speech-in-noise metrics.
What it means for you today: There is no approved synapse-repair drug yet. For now, hearing aids with excellent speech-in-noise features, assistive microphones, and auditory training remain the best tools. If speech-in-noise is your main pain point, ask an audiologist about advanced features and accessories that beam the speaker’s voice directly to your ears.
Frontier #2: Regrowing hair cells—turning back the cochlear clock
Birds and some fish can regrow cochlear hair cells. Humans can’t—at least not naturally. Could we coax supporting cells in the cochlea to become new hair cells?
What scientists are trying
- Gene activation (Atoh1) and signaling pathways: Atoh1 is a master switch for hair-cell development. Experimental approaches try to nudge cochlear supporting cells to re-enter a “developmental mode.”
- Notch pathway modulation: The Notch pathway helps determine which cells become hair cells versus supporting cells. Inhibitors may shift that balance.
- Progenitor cell activation: Drugs designed to awaken dormant regenerative capacity in the human cochlea.
Where the evidence stands
- In animals: Researchers have created new hair-cell–like cells and improved hearing in certain models, but translating to humans is complex.
- In humans: Early trials of some regenerative small molecules did not meet primary endpoints for meaningful hearing improvement, and programs paused. We learned a lot about dosing, delivery, and how to measure real-world benefit—not a cure yet.
Key challenges: The inner ear is tiny; getting drugs to the right spot and keeping them there is hard. Also, even if new hair cells appear, they must connect to the right neurons and function in concert with the rest of the inner ear. That wiring challenge is nontrivial.
What it means for you today: No approved hair-cell regeneration therapy exists for age-related or noise-induced loss. Hearing aids and, for severe losses, cochlear implants remain the gold standard for restoring access to speech.
Frontier #3: Gene therapy—fixing the root cause in specific inherited losses
Some forms of hearing loss are caused by a single gene that’s not working. If you can deliver a healthy copy of that gene to the right inner-ear cells, you might restore function—especially if treated early.
What scientists are trying
- AAV (adeno-associated virus) delivery: A well-known viral vector used to carry healthy genes to inner-ear cells.
- Targets like OTOF: Otoferlin (OTOF) mutations cause a form of congenital deafness with otherwise intact cochlear structures. Replacing the faulty gene is a logical strategy.
Where the evidence stands
- Early human data are promising: Recent reports show partial restoration of hearing in some children with OTOF-related deafness after inner-ear gene therapy. Multiple trials are ongoing internationally to confirm safety, dosing, and durability.
- Who might benefit: Right now, gene therapies are aimed at specific, confirmed genetic diagnoses—often in children—where the inner ear is structurally present but missing a critical protein.
Key challenges: The inner ear is delicate. Delivery typically involves a surgical injection near the round window of the cochlea. Large genes may not fit into standard vectors, and immune responses, durability, and long-term safety need careful study.
What it means for you today: If hearing loss runs in your family or began early in life, and you’re curious about gene therapy, genetic testing via a medical provider can clarify whether a known single-gene cause is present. For adults with age-related or noise-related loss, gene therapy is not yet applicable.
Hype filter: How to read hearing “breakthrough” headlines
- Model matters: A mouse gaining hearing isn’t the same as a human with decades-old damage improving speech understanding in a busy restaurant.
- Endpoints matter: “Improved thresholds at one frequency” is not the same as “I catch every word at my granddaughter’s recital.” Look for speech-in-noise outcomes, not just pure-tone audiograms.
- Time matters: Early case reports can be real and exciting, yet long-term durability and safety are still unknown.
- Population matters: A therapy for a specific genetic mutation may not generalize to age-related or noise-induced loss.
What you can do now while the science matures
Make today’s hearing count
- Use well-fit hearing aids: They reduce listening effort, preserve social connection, and are linked with better health outcomes. Ask your audiologist about real-ear measurements, directional microphones, and accessories that improve speech-in-noise.
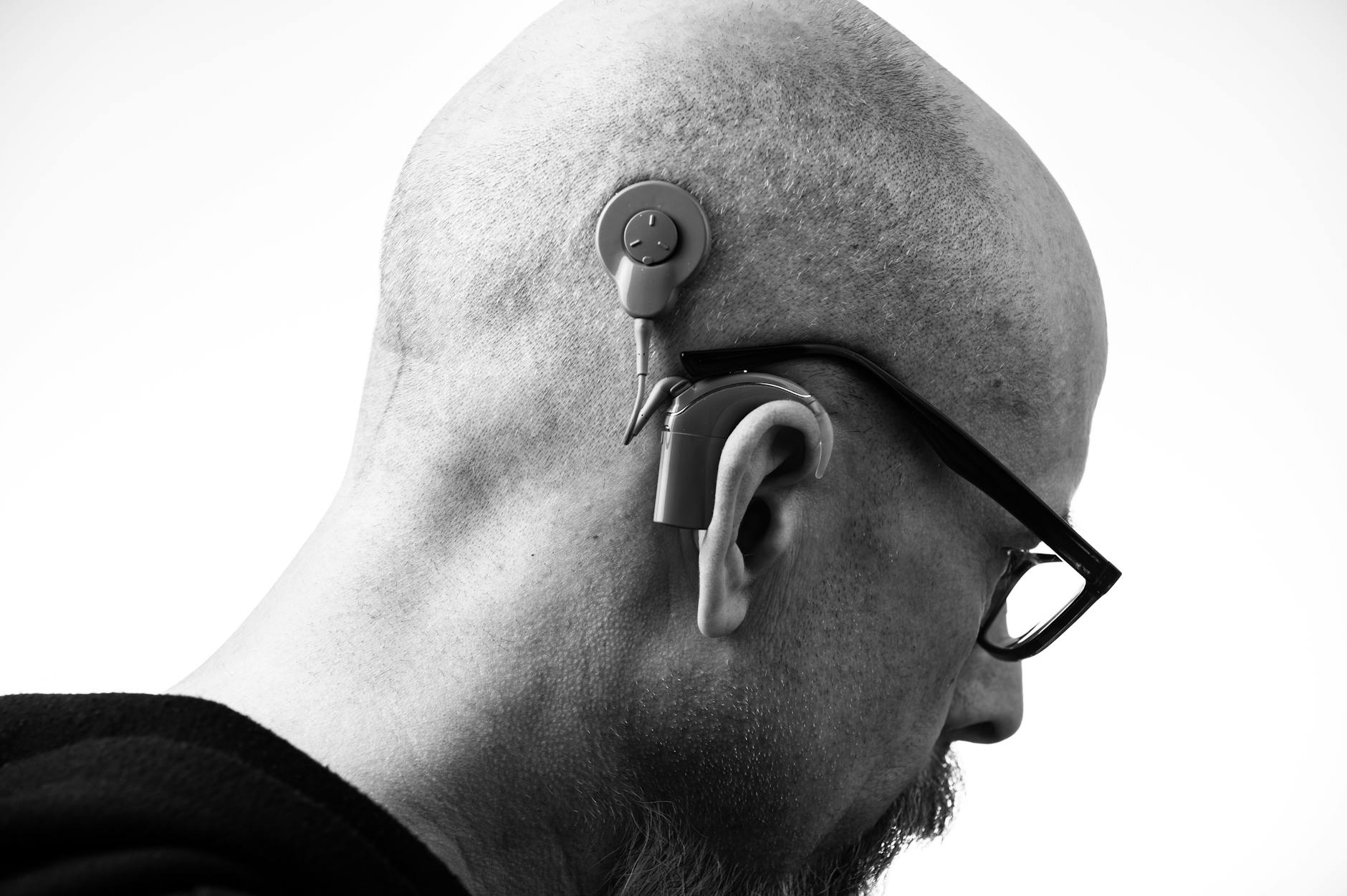
- Consider cochlear implants if appropriate: For severe-to-profound losses, modern implants provide life-changing access to speech. Many centers also study ways to preserve and even enhance residual hearing around the implant.
- Train the brain: Auditory training and consistent hearing-aid use can sharpen speech understanding over time.
Protect what you have
- Dial down the dB: Wear hearing protection for loud work, concerts, yard tools, or workouts with amplified music.
- Mind your health: Manage cardiovascular risk factors, diabetes, and sleep—your inner ear thrives on healthy blood flow and low inflammation.
- Medication check: Ask your healthcare team about potential ototoxicity if you’re starting new medications, especially certain chemotherapy or high-dose antibiotics. Never stop a prescribed drug without medical advice.
Interested in research?
- Talk to an audiologist or ENT: They can help you understand your specific hearing profile and whether a clinical trial might fit your situation.
- Check reputable registries: ClinicalTrials.gov lists studies of synapse repair, hair-cell regeneration, and gene therapy. Read inclusion criteria carefully.
- Ask smart questions: What is the goal (safety vs. efficacy)? How is the drug delivered? What outcomes are measured? What are the risks and time commitments?
Realistic timelines—and real hope
We’re moving from “can we do anything?” to “can we help specific people in specific ways?” That’s progress. In the near term:
- Gene therapy may expand for certain single-gene causes in carefully selected patients, likely starting with children.
- Synapse-focused therapies will keep evolving as scientists refine delivery and measurement.
- Hair-cell regeneration remains the toughest nut to crack, but lessons from early trials are shaping smarter next attempts.
Meanwhile, the hearing tools we already have—smart hearing aids, remote microphones, captioning, and cochlear implants—are powerful. Getting the most from them now doesn’t close the door on future therapies; it keeps your brain engaged with sound and your life connected.
Gentle nudge: If you’re wondering where you stand, schedule a comprehensive hearing evaluation. A tailored plan today plus a clear view of the research horizon is a winning combo.
Further Reading
- Can We Regrow Hearing? The Real State of Hair Cell Regeneration (Research) - Hearing Damage, Measured in a Drop: The Emerging Science of Ear Biomarkers (Research) - Your Heart, Your Hearing: The Cardiometabolic Link You Can’t Afford to Ignore (Research) - Long COVID and Your Hearing: What We Know (and What to Do Next) (Research)Frequently Asked Questions
Will hair-cell regeneration or synapse repair help my tinnitus?
It’s possible, but unproven. Tinnitus often relates to changes in the auditory system after hearing damage, including hair cells, synapses, and brain networks. If a future therapy truly restores peripheral function, some people might notice tinnitus improve, but results could vary. Right now, evidence-based tinnitus care includes sound therapy, counseling approaches like CBT, optimizing hearing aid settings, and stress and sleep support. An audiologist can personalize options.
How close are we to a therapy for age-related or noise-induced hearing loss?
There’s no approved regenerative or synapse-repair therapy yet. Early gene therapy is showing benefits in specific childhood genetic conditions, which is encouraging for the field. For common adult hearing loss, expect incremental progress over several years—better diagnostics, more targeted trials, and possibly niche therapies first—rather than an overnight cure.
If I get a cochlear implant now, does that prevent me from trying future inner-ear therapies?
Not necessarily, but it depends on the therapy and your inner-ear status. Many implant candidates have little residual hair-cell function left. Some research is exploring ways to preserve or even enhance residual hearing around implants. If you’re weighing an implant, discuss long-term considerations with your cochlear implant team. Don’t delay proven treatment in hopes of an unproven future option without guidance from your clinicians.
Should I get genetic testing for hearing loss?
If hearing loss began early in life, runs in your family, or your clinician suspects a single-gene cause, genetic testing can clarify your diagnosis and potential eligibility for research. Testing is most useful when done with a genetics-informed clinician who can interpret results and discuss implications.